Just Add Water: Possible Key to Energy Storage for Electric Vehicles
 |
SEE ALSO: Electric Vehicles - Solution or Diversion?
NISKAYUNA, N.Y. August 28, 2013; It's a little more complex than making instant oatmeal, but scientists from GE and Lawrence Berkeley National Laboratory (Berkeley Lab) may have just the recipe for next-generation electric vehicle (EV) batteries that achieve desired driving range and cost for consumers.
The GE/Berkeley Lab team is developing a water-based, flow battery capable of more than just traditional, stationary energy storage. Chemistries that GE scientists are developing will enable a flow battery that derives its power from a novel electro-chemical reaction that all resides safely in a bath of water.
Grigorii Soloveichik, project leader on the water-based flow battery project at GE Global Research and director of the GE-led Energy Frontier Research Center (EFRC), said, "We're excited about the impact this new technology could have on electric vehicles, especially as it relates to cost and the need to recharge. Our flow battery could be just one-fourth the price of car batteries on the market today, while enabling roughly three-times the current driving range. The DOE wants a battery that can power a car for 240 miles; we think we can exceed that."
This Labor Day weekend, AAA estimates 34.1 million drivers will travel 50 miles or more. With a 240 mile driving range, many would be able to drive their entire weekend on a single flow battery charge, saving families money while reducing emissions.
The work on this project will greatly benefit from the skills and knowledge acquired from GE's ongoing leadership in the U.S. Dept. of Energy's EFRC program. GE's EFRC was designed specifically for building a fundamental base for next-generation energy storage technologies. GE scientists will be working closely with team from Berkeley Lab on development of this battery technology.
â"The opportunity to expand our collaboration with GE from the EFRC to applied research under ARPA-E is of great interest," said Adam Weber, Berkeley Lab Staff Scientist and PI for this project. "We have had great success in developing high-power traditional flow batteries, and the possibility of using that expertise for a high-energy flow battery is quite compelling."
Aside from offering significant advantages in terms of cost and range, the flow battery GE is researching would offer safety improvements over batteries used in cars today, and could be easily integrated into current car designs; both stated goals of ARPA-E's RANGE program.
The proposed flow battery uses water-based solutions of inorganic chemicals that are capable of transferring more than one electron, providing high-energy density. Discharge and re-charge of such flow batteries occur in electrochemical cells separated from energy storing tanks, which makes them safer.
Over the next year, the GE/Berkeley Lab team will demonstrate feasibility of this new battery concept and develop a working prototype.
MIT: What is a flow battery?
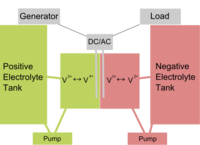
A flow battery is a special type of rechargeable battery in which two liquids with opposite electric charge (electrolytes) exchange ions, converting chemical energy directly into electricity. The electrolytes are usually separated by a thin membrane that lets them exchange ions without mixing.
The electrolytes are stored separately from the cell itself, in two big tanks, and the electrolyte is pumped into the cell as needed. This means the system can be scaled easily, simply by changing the size of the tank. Doing so can produce systems of vastly different capabilities, from a few kWh up to several MWh.
Scalability aside, flow batteries come with many more perks: they can stay idle for long periods of time without losing their charge, they have a quick response time, and they can charge and discharge quickly just by replacing the electrolyte fluid. For these reasons, over the past few years some people have advanced them as a way to quickly refuel electric cars.
On the flipside, flow batteries are more complicated than standard batteries, each requiring their own system of pumps and sensors; moreover, energy densities are usually lower than those of your standard Li-ion battery.
A battery for the future
The main challenge in developing an effective flow battery has been to find a good balance between performance and costs. The electrolytes used are typically not very expensive, but they tend to eat away at the costly membrane, shortening the battery's lifetime. The MIT team's solution circumvents the issue in perhaps the most elegant of ways – by taking out the membrane altogether.
The small flow battery prototype built by the researchers uses a curious phenomenon in fluid dynamics called laminar flow: if both liquids are kept at low enough speeds and other conditions are satisfied, the two electrolytes won't mix, making the membrane superfluous.
Pumping liquid bromine over a graphite cathod and hydrobromic acid and hydrogen gas over a porous anode, the researchers created a reservoir of free electrons that can be discharged or released at will.
While other teams had attempted a membraneless design before, this is the first one in which the battery can be recharged as well as discharged. Their flow battery produced up to 0.795 watts per square centimeter: that's three times as much as other membraneless systems, and about 10 times higher than most lithium-ion batteries.
Future developments
The researchers also generalized the manufacturing parameters of the flow battery, by creating a mathematical model that they can use to optimize the system and eventually build larger-scale devices that are better suited to grid applications.
Previous membraneless systems have been largely unpractical, but the scaled-up version of the device could have a substantial real-word impact because it could be used to produce energy for a very competitive US$100 per kilowatt-hour. "Most systems are easily an order of magnitude higher, and no one’s ever built anything at that price," says William Braff, who was part of the research team.
One area in which this technology could be put to good use is the storage of renewable energy. Since sunlight and wind are highly unpredictable power sources in the short term, being able to store large quantities of clean energy to use as a buffer is essential if green energy is going to continue to satisfy a larger and larger portion of our energy needs.
The team's research appears in the journal Nature Communications.
Sources: MIT, Electropaedia About Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory addresses the world's most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab's scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy's Office of Science. For more, visit www.lbl.gov.